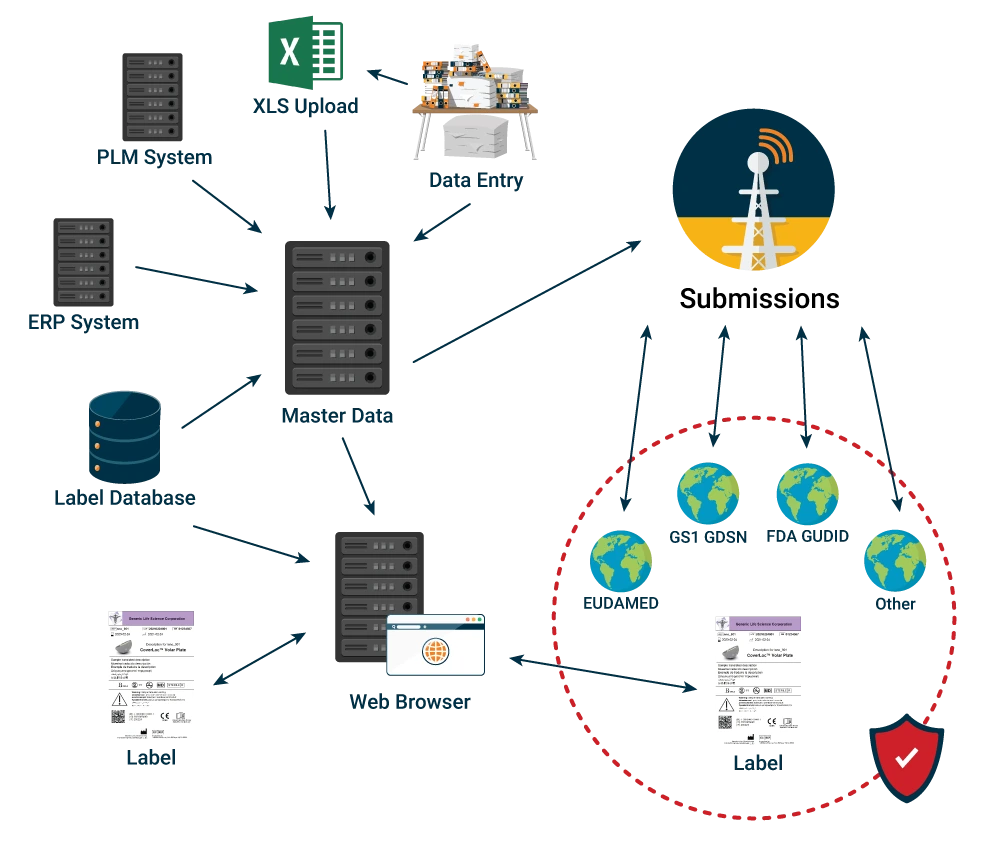
Regulatory Data Submissions
Complete In-House Global Regulatory Submissions for Life Sciences Manufacturers
Provide your business with the capability of conducting its global regulatory submissions in-house. After a cleansed and verified repository of data has been achieved via ROBAR Master Data Management, any number of external entities can become the recipient of their data segment, transforming the data stored via the ROBAR global submissions capability.
Select Customers

























Keeping Up With Regulations Worldwide
New UDI submissions regulations and updates from India, Brazil, Turkey, China, Argentina, Canada, and other regulatory bodies are on their way. As members of the Medtech EU MDR working group for Eudamed Machine-to-machine (M2M) communications, Innovatum employees are Eudamed experts and well versed in the needs of other bodies requiring global regulatory submissions. We are continually guiding and staying on top of what is needed in the global regulatory space.
Maximize Data Reuse
Often the attributes required by one regulatory body are very similar to the attributes of another. Allow reuse of data that is common to requirements of different regulatory bodies, while tracking and making regulation specific data available as needed. The system transforms the data as required by each receiving regulatory body.
Flexible Data Access Capabilities
Support the ability to select multiple entries from a list (e.g., configure kit components or multiple patent numbers). Provide immediate and easy access to records in various stages of preparation and submission and make data available for publication in Excel, documents, and cloud based regulatory submissions systems like GUDID and Eudamed.
Provide Regulatory Significant Data with Submissions Module
Select blocks of records that meet filtering criteria (e.g., internally approved status, family, product name, device class, etc.), submit to regulatory bodies, and receive vital partner system acknowledgement including error feedback for correcting records before resubmission.
Global Submissions Technologies
Plug-in architecture minimizes validation efforts as new submissions requirements are released by regulatory bodies. Our plug-ins provide automation of communication protocol configuration and data mapping to trading partners’ databases such as GUDID, GS1 GDSN Datapool provider, etc., using the required AS2, HL7, or XML.
Eliminate Reliance on A Third Party
Gaining complete control over your submissions means that acknowledgement information will return quickly, so correcting and resubmitting is expedited and efficient. As regulatory requirements compound, you have your data all in place and ready to be manipulated as required by using ROBAR.
We Work With Organizations Around the World
We provide complete systems, modular systems, consulting, training, and exemplary 24/7 support services across all borders and time zones.