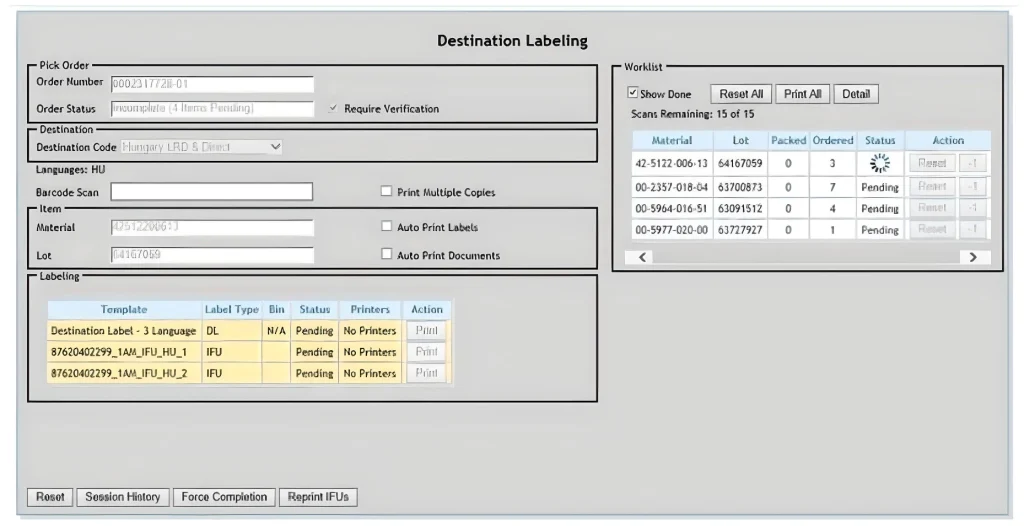
Destination Labeling
Master Regionally Specific Labeling with ROBAR Destination Labeling
Destination labeling or labeling localization, just-in-time labeling, print-on-demand, or country/regionally specific labeling—however you name it, we have you covered. ROBAR delivers the most powerful answer to shrinking label real estate where content requirements for language translations, regulatory and economic content, and symbologies on the label are expanding.
Select Customers

























Automatic Labeling For Each And Every Destination
Regardless of the destination of your product, ROBAR Destination Labeling ensures that the right information is on the right package at the right time. Associations are made between items, destinations, languages, special symbols, etc. in the ROBAR Database and everything flows automatically from there.
Exploit the Power of Barcode Triggered Linkages
Make and exploit one-to-many and many-to-many associations between items, templates, documents, regions, countries, or CLAD (Core Languages All Destinations) in ROBAR Master Data Management.
Integrate Your IFU Management
Upload and store IFUs (Instructions for Use) and other content documents within ROBAR Master Data Management. Create linkages of IFUs to item versions and destinations. These associations can then be used to print appropriate documents for specific locations.
A Browser-Based Page to Read Order
Fulfillment Pick-Lists
Print documents based on user input or scan and drive multiple printers simultaneously. An internet connection is all that is needed wherever the printing takes place. Screen based prompts report on printing progress for all printer jobs including IFUs and labels.
Handle Markedly Increasing Trade
Requirements Around the World
Developments in technology make destination labeling easier to implement. The UDI, and the powerful information it unlocks within enterprise systems, will be key factors in implementing a robust and intelligent, regionally specific labeling system.
Solve Regionally Specific Challenges
Print specific registration numbers in China and Russia, local contact information for importers and other economic operators, and small durable implant cards in local languages. Provide MSRP in Rupees for products sold in India or use regionally specific symbols for USA versus EU.
Do It All with One System
From data and design to global production printing all the way to retirement, ROBAR handles it all. Regulatory data submissions, serialization, and 100% Print Inspection completely round out the picture.

Designed for Regionally Specific Labeling - ROBAR Destination Labeling White Paper
Destination labeling, also known as localization or country specific labeling is the process by which appropriate labels that contain necessary content are printed after the manufacturing and packaging steps, often during customer order fulfillment.
We Work With Organizations Around the World
We provide complete systems, modular systems, consulting, training, and exemplary 24/7 support services across all borders and time zones.