Regulatory Compliance
Keeping Companies Compliant - Superior Software Paired with Extraordinary Support
Automating processes improves efficiency while reducing risks and costs. No matter the compliance requirement, abiding by and documenting proof of compliance is much easier with a system that is purpose built for compliance activities. With ROBAR, building in regulatory compliance becomes a foregone conclusion. Whether you are working on regulated labeling, regulatory data management, and/or submissions, compliance capability is always baked in.
Select Customers


























Compliance Capability Baked In
The most efficient way to achieve and demonstrate compliance is to start with systems that have been designed and built from the ground up to achieve and maintain demonstrable compliance. Use systems with: Controlled access security, digital signature capture, automatic versioning, 21 CFR Part 11 reporting, Annex 11, secure reprints, before and after data values, change control… the list goes on and on and Innovatum provides it all within ROBAR.
FDA 21 CFR Part 11
All life sciences manufacturers employing the use of computers in their GxP processes need to be 21 CFR Part 11 compliant. Once validated for your specific environment, ROBAR offers complete 21 CFR Part 11 compliance for your compliance related activities.
EU Annex 11
Compliance requirements for FDA Title 21 CFR Part 11 and EU Annex 11 are equivalent, and ROBAR is compliance capable for both.
Compliance From The Outset
UDI
As regulatory bodies around the world put their spin on Unique Device Identification and the way the UDI barcoding will be used, ROBAR is ready. A plug-in based approach ensures global scalability with minimal additional validation.
EU MDR IVDR
For Innovatum’s ROBAR, compliance with EU MDR and IVDR is merely an extension of using already proven US FDA UDI solutions. As Eudamed requirements evolve, the abilities of ROBAR evolve alongside them.
ISO 9001:2015
Realizing the importance of maintaining a Quality Management System for software development, delivery, and support, Innovatum maintains certification. As such, Innovatum is committed to providing products and services of the highest quality, consistent with the needs of our customers and applicable regulatory requirements. “Our Quality Policy is to continually strive to attain the highest level of customer satisfaction by implementing management tools and practices that will allow us to monitor feedback and take actions to continually improve and satisfy the needs of our customers. Our Quality objective is to strive for 100% Customer Satisfaction.”
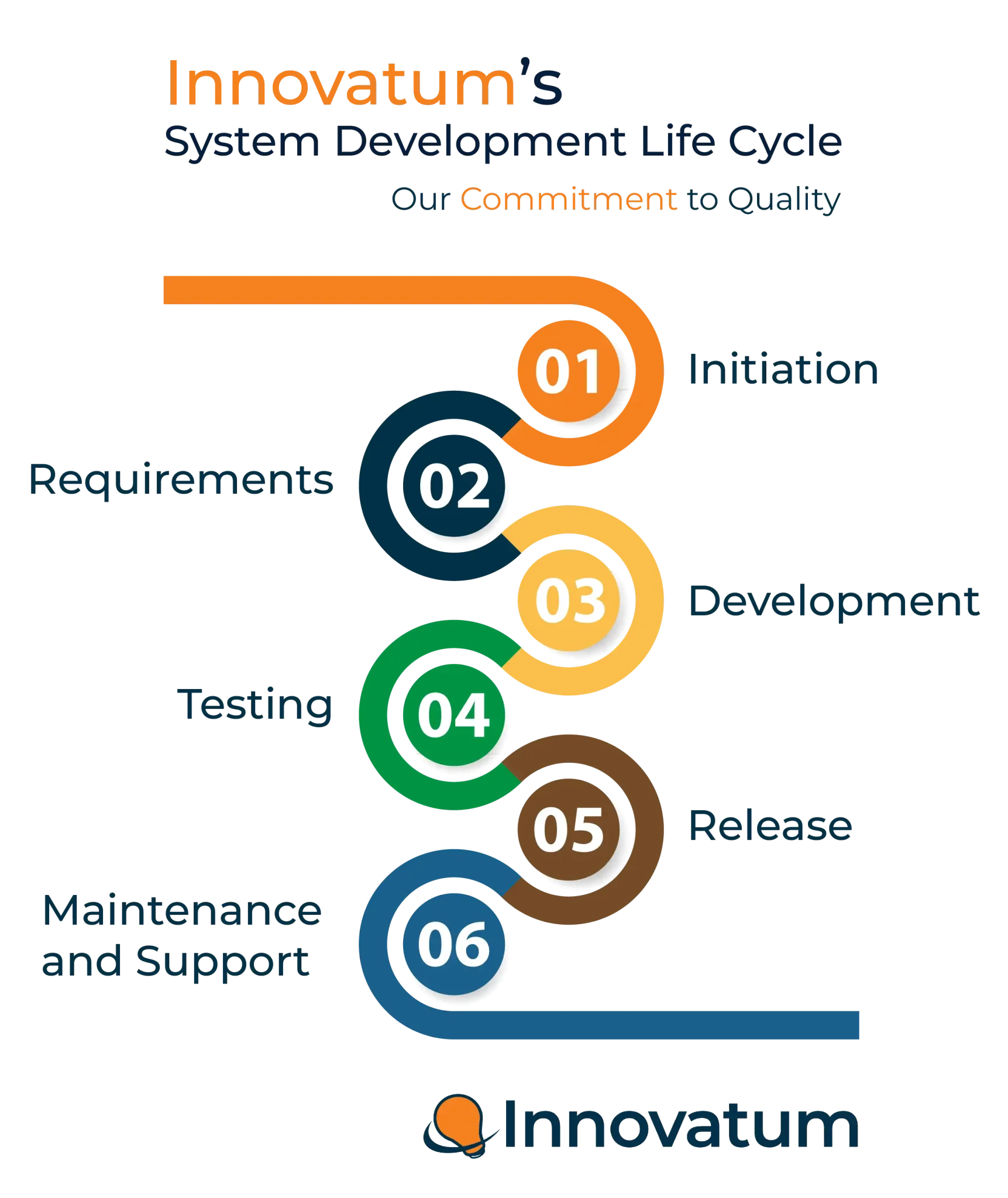
GxP Compliance
Innovatum conducts regular trainings for its employees and follows GAMP 5 and a stringent SDLC process for its software development. We thoroughly test every release and perform regression testing on all enhancements in a branch and trunk approach and validation documentation is made available.
US FDA Drug Supply Chain Security Act (DSCSA)
Basic elements of the act include managing barcode driven packaging aggregation, interoperability with a host of other systems, and making traceability information available to other systems across distribution channels for surveillance tie-ins. ROBAR enables them all.
We Work With Organizations Around the World
We provide complete systems, modular systems, consulting, training, and exemplary 24/7 support services across all borders and time zones.