Regulatory and Product Master Data Management
The Most Complete and Powerful 21 CFR Compliant Labeling Content System Available for Life Sciences Manufacturers
Serving as pass through, additional repository or source of truth, ROBAR enables auditable, easy review, approval, and change management of related data content in a 21 CFR compliance capable manner.
Select Customers

























Exceptional Regulatory Data Management
100% of our customers have agreed to be references over our two decades of providing services and consultation. Our solid reputation for excellent consulting and, arguably, the best support in the industry, has allowed us bring our end to end enterprise labeling system to its current impressive and elevated state.
Powerful and Flexible Enterprise Labeling Database
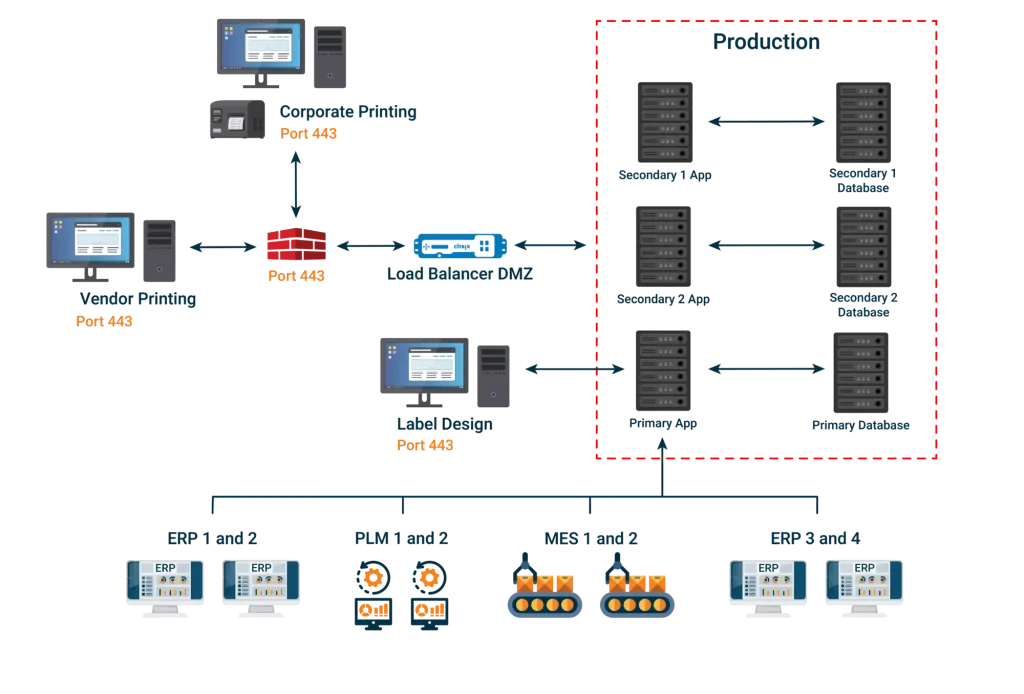
ROBAR integrates information from different sources of truth as a pass through for data from enterprise systems and serves as a repository for data that does not presently have a home. It provides clean pulls and pushes of enterprise labeling data regardless of the systems involved.
Virtually Limitless
ROBAR provides a virtually limitless, configurable database, which does not require re-validation after configuration changes (e.g. the addition of fields, grouping of fields, etc.) are made. It is a master data management system that grows along with your company.
Access Through a Browser
Our browser based system allows for input, editing, approvals, and flexible usage in a variety of areas ranging from labeling content to global submissions for various forms of UDI, eIFU management, 100% label inspection, and more.
Control Content, Digital Assets, Approvals, and Usage Globally
Provide controlled access to stakeholders wherever they may be. Enable collaborative work by giving departments access the only the data they need to see, make changes to, or approve.
Track the Granular History of Changes to Each Data Element
Although required by 21 CFR, this kind of granular history is rarely delivered by any database with the exception of ROBAR, despite this information being useful for understanding where, when, and by whom or what (other systems) changes to data are being made. Users may view before and after values complete with timestamps and digital signatures in detailed change history reports.
Overcome Siloing
ROBAR eliminates redundant sources of data and approvals in different places, as well as the need to keep multiple databases and/or spreadsheets in sync. Groups of people can access and manage the data they are directly responsible for.
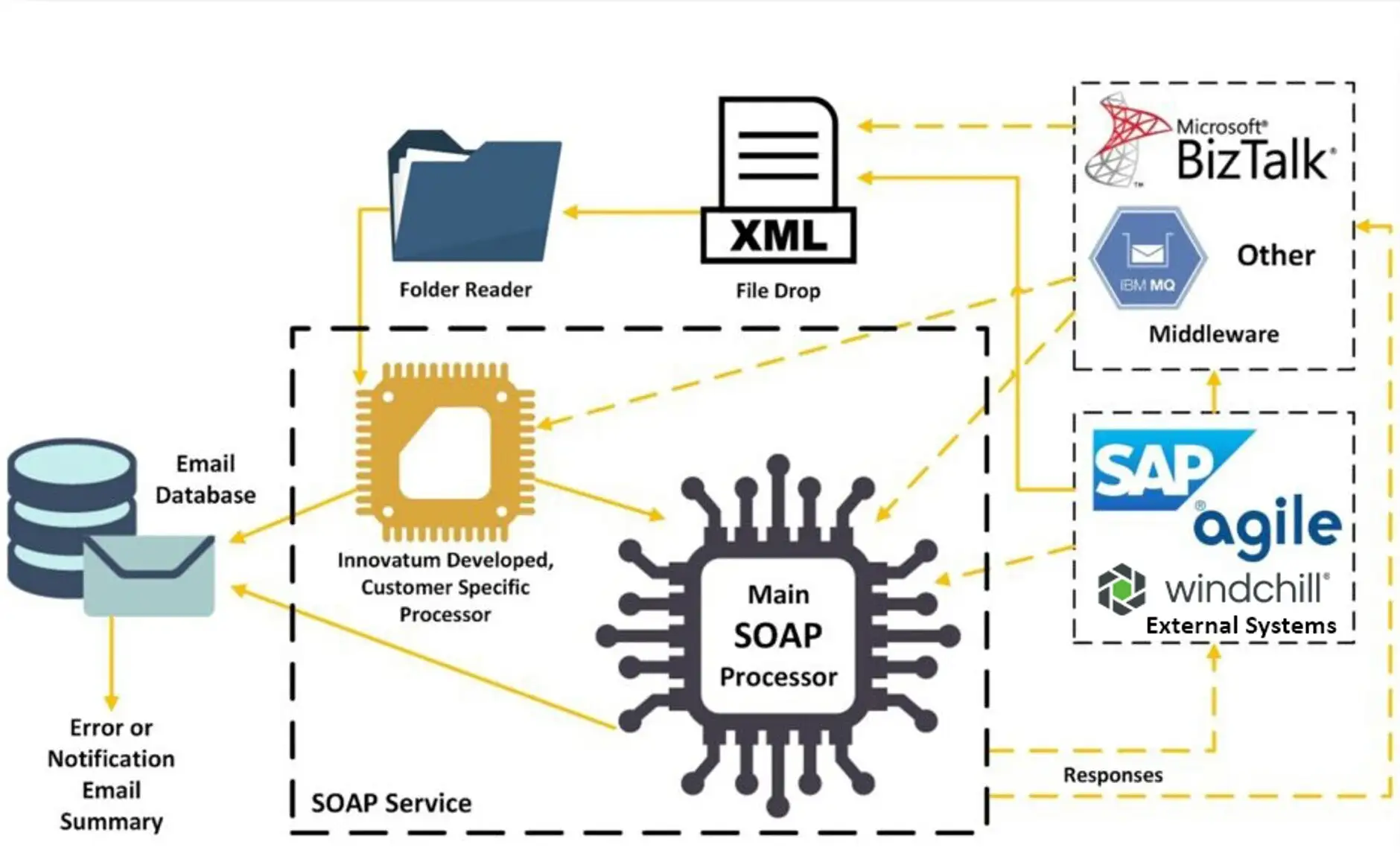
Exceptional Connectivity to Enterprise Systems for Regulatory Data Integration
Exceptional Capabilities
Easily drive tightly controlled labeling content specific to destinations, while complying with regulations and data submissions requirements of various regulatory bodies around the globe. The data content within the ROBAR database can be used for anything from product and package labeling to regulatory submissions, GDSN Data Pool upload, eIFU hosting, ePIC and medical implant cards, 100% label inspection, and more.
Maximize Data Reuse
Often the attributes required by one regulatory body are very similar to the attributes of another, allowing the reuse of data that is common to requirements of different regulatory bodies while tracking and making regulation specific data available as needed.
Flexible Data Access Capabilities
ROBAR supports the ability to select multiple entries from a list (e.g., configure kit components or multiple patent numbers) and provides immediate and easy access to records in various stages of preparation and submission. It makes data available for publication in Excel and documents including, but not limited to, labels or cloud based regulatory submissions systems like GUDID, Eudamed, and others that are on the way from other regulatory bodies.
Enforce Security
Enforce business unit level and column level security and enable each department to manage their own data.
Multiple Data Entry Points
Control browser based access for manual data entry and/or data maintenance and approvals. Receive variable data that is being managed and stored within other systems and automatically version control with electronic signatures and Part 11 compliance.
Unmatched Integration Capabilities
ROBAR offers more integration options than any other labeling system and includes both pull and push capabilities.
We Work With Organizations Around the World
We provide complete systems, modular systems, consulting, training, and exemplary 24/7 support services across all borders and time zones.





