End to End Life Sciences Labeling Solutions
Hosted or on Premises Labeling, Regulatory Data Management, Submissions, and All Related Services
Enterprise Labeling
Innovatum’s ROBAR Enterprise Label Management System for FDA-regulated pharmaceutical, medical device, and biotechnology manufacturers.
Regulatory And Master Data Management
ROBAR MDM can be used to effectively manage regulatory data as a RIMS and provide it to ROBAR Labeling along with PIMS data for use in label printing.
Regulatory Data Submissions
Managing the bidirectional process of regulatory data submissions and approvals using requisite technologies such as HL7 and others.
Select Customers


























Superior Software Extraordinary Support
Superior Software
Extraordinary Support
The ROBAR Advantage
Don't Customize... Configure
All enhancements become part of the product. No back applying of enhancements are required as part of an upgrade. Upgrades are included as part of maintenance.
Upgrades Included As A Part Of Maintenance
All Innovatum customers who are current with their maintenance are presented with the option of upgrading to newer versions with additional functionality at no extra charge for the newer software version.
All In One Solution
That includes data maintenance, labeling and regulatory compliance.
We offer
Best Support
In Industry
Unmatched issue resolution times and customer satisfaction ratings
Adobe Suite Artwork
Management
Versions & manages native Adobe files- associates them with items
Unmatched Risk
Reduction
Commonly referred to as a quality management system for labeling
True Enterprise
Collaboration
Browser based with unlimited access licensing for your complete supply chain
Compliance Capability
Built-In
Completely 21 CFR and Annex 11 capable with reporting
Manages Data and
Regulatory Submissions
GUDID, EUDAMED, GS1 Data Pool, Others

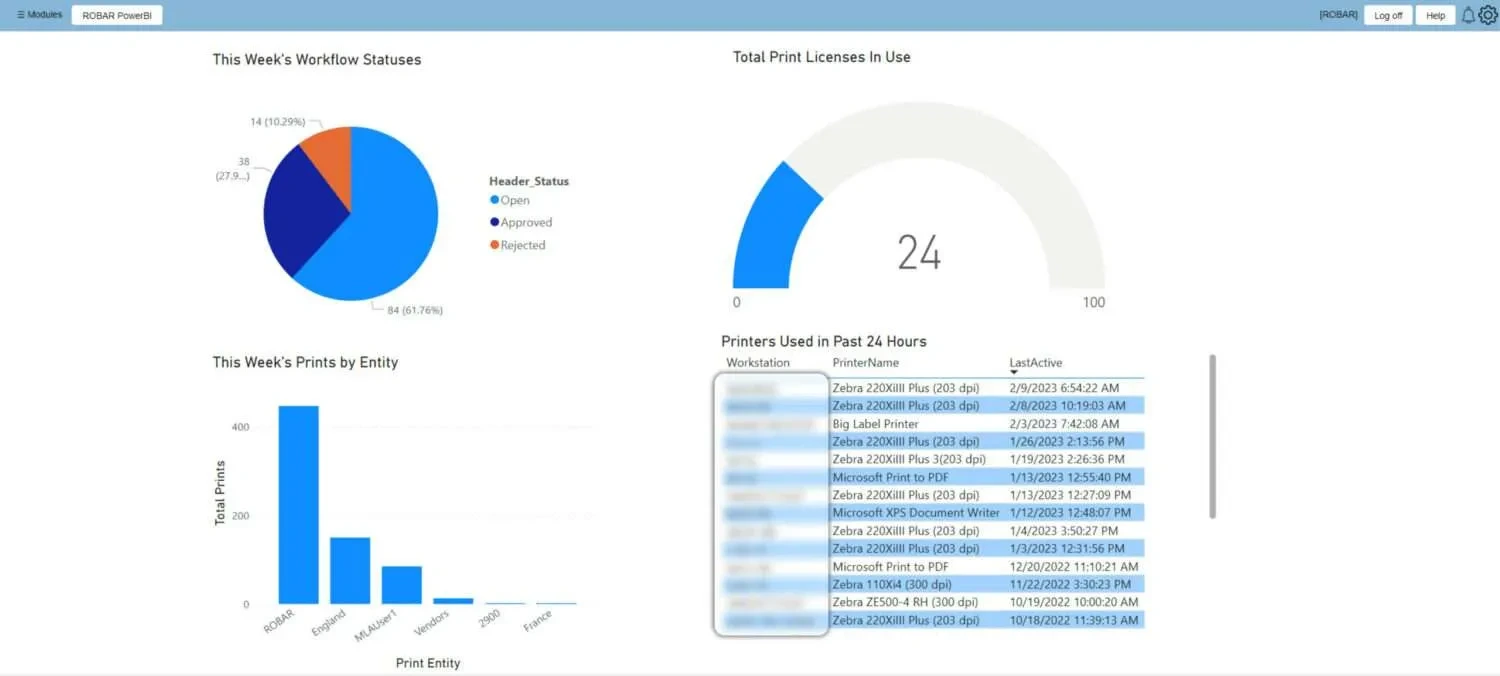
System Dashboard
We Work With Organizations Around the World
We provide complete systems, modular systems, consulting, training, and exemplary 24/7 support services across all borders and time zones.
Our Integrated Solution
Innovatum’s ROBAR provides many powerful capabilities for UDI/MDR/IVDR/Eudamed and is delivered with consulting, implementation, and validation assistance. As a true end-to-end regulated labeling system provider, Innovatum has been a top innovator in life sciences labeling for over 25 years. Innovatum’s fully configurable and easily validatable RIMS/PIMS/MDM labeling systems are easily expandable to meet future regulatory needs without involving the IT department. Additional modular capabilities include 100% label inspection and elFU management with hosting.
3-10 Seconds
Average First Label Out
6,000+
Natively Supported Printers
In Minutes
Avg Support Issue Resolution
100%
Of Customers Have Agreed To Be References
30,000 to 1
30,000 SKUs Sharing One Label Template Format